Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank


Suzhou Evermed Biomedical Co., Ltd
We are professional supplier of Therapeutic Drug Monitoring (TDM),Life Insurance Testing,Steroid / Endocrine Testing,Veterinary / Companion Animal Testing.
Business Type:Manufacturer
Country/Region:China
Ddu Verified
HOT Rank

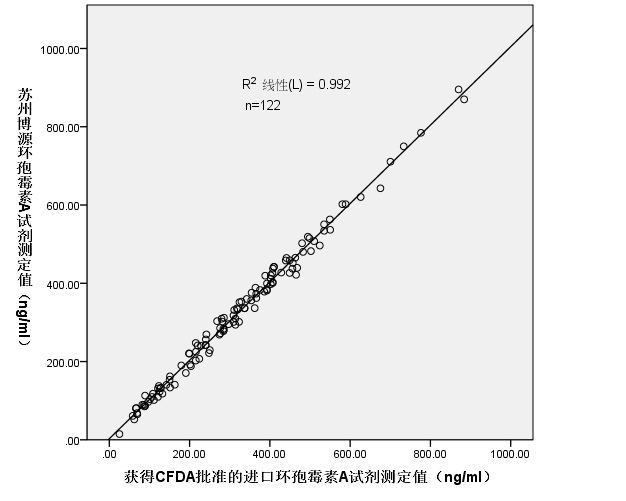
Evermed Cyclosporine A Homogeneous Enzyme Immunoassay | |
* CLINICAL IMPLICATIONS | |
Cyclosporine A (CsA) is a hydrophobic cyclic undecapeptide of fungal origin with immunosuppressive properties [1-2]. CsA is an effective drug for the treatment of certain autoimmune diseases and prevention of tissue rejection following organ transplantation. CsA therapy has optimal safety and efficacy over a narrow range of concentrations and may lead to a number of adverse effects [3-4]. The most critical adverse effects are organ rejection from inadequate dosing, or nephrotoxicity and hepatotoxicity when drug concentration becomes too high. CsA is administered either orally or intravenously. Since absorption and hepatic metabolism of the drug are highly variable from patient to patient, there is a poor correlation of blood levels with the administered dose. It is essential to monitor CsA in organ transplantation patients to achieve optimal immunosuppressive effects[5-6]. | |
* TEST SAMPLE TYLE | |
Whole Blood | |
* KEY POINTS | |
| |
* REFERENCES | |
[1]Borel. J.F., Cyclosporin A - present experimental status, Transplant Proc 13: 344-348, (1981). [2]Schreiber, S.L., Chemistry and biology of the immunophilins and their immunosuppressive ligands, Science 251: 283-287, (1991).[3]Sells, R.A., Transplantation Proceedings 15: 2495, (1983). [4]Alberchtsen, D., Soedal, G., Berg, K.J., Bondevik, H., Brekke, I.B., Fauchald, P., Jakobsen, A., Opedal, B.R. Rugstand, H.E. Thorsby, E., et. al., Cyclosporine in Living Related and Cardaveric Renal Transplantation. [5]Morris, R.G., Russ, G.R., Cervieeli, M.J. et al., Comparison of trough, 2-hour, and limited AUC blood sampling for monitoring cyclosporin Neoral® at day 7 post-renal transplantation and incidence of rejection in the first month. Ther. Drug Monit. 24(4): 479-485, (2002). [6]Andrews, D.J., and Cramb, R. Cyclosporin: revisions in monitoring gujidelines and review of current analytic methods. Ann. CLin. Biochem. 39: 424-435, (2002). |